Midterm Exam 2 Study Guide
The second midterm exam will cover Lectures 8-13 & Chapters 6-8 in the textbook. See below for a comprehensive summary of the topics included on the exam. While the end of chapter problems in the textbook are always a great place to start, the REVIEW MATERIALS page has many additional practice problems and their answer keys. Use the iClicker Questions to review common misconceptions & typical mistakes. Remember, the annotated lecture slides are also posted to the course schedule. If you need additional review, you can always consult the video tutorials and animations posted for each lecture (also found on the course schedule).
Disclaimer: Practice Exams from previous semesters may cover more or less material than the current semester. No two problems will likely ever be the same. Avoid memorizing how to solve a specific problem, and instead focus on the concepts and logic necessary to problem solve.
- Practice Exam Download Practice Exam (Fall 2013) & ANSWER KEY Download ANSWER KEY
- Practice Exam Download Practice Exam (Fall 2014) & ANSWER KEY Download ANSWER KEY
- Practice Exam Download Practice Exam (Summer 2015) & ANSWER KEY Download ANSWER KEY
- Practice Exam Download Practice Exam (Spring 2016) & ANSWER KEY Download ANSWER KEY
- Nomenclature:
- Naming Ionic Compounds & writing their formulas:
- Predict the charge of a monoatomic ion based on its position in the periodic table
- Use Roman Numerals to specify charge of transition metals & other metals with VARIABLE charges:
- To avoid redundancy, charge should not be specified for metals in columns 1A, 2A or aluminum
- Be sure formula is written so that the cations balance with the anions to generate a neutral compound (charge = 0)
- Commit these polyatomic ions to memory
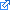
- Acidic Compounds:
- Oxoacids:
- -ate suffix is converted to -ic acid
- -ite suffix is converted to -ous acid
- Acids without oxygen:
- hydro- prefix is used
- -ide suffix is converted to -ic acid
- Naming Molecular, Covalent Compounds and writing their formulas:
- Know rules for naming with the prefixes (mono-, di-, tri, tetra-, etc.) to specify the number of each element
- Commit to memory the prefixes specifying 1-10:
- mono-
- di-
- tri-
- tetra-
- penta-
- hexa-
- hepta-
- octa-
- nona-
- deca-
EMPIRICAL & MOLECULAR FORMULAS:
- Know the difference between empirical and molecular formulas
- Know how to calculate empirical formulas from percent composition by mass
- Know how to calculate molecular formulas starting from the empirical formula and molar mass
CHEMICAL EQUATIONS:
- Prerequisite knowledge: Nomenclature of ionic and covalent compounds
- Don't forget the polyatomic ions!
- Know the difference between the stoichiometric coefficients (the numbers specifying how many of each reactant and product are involved in the reaction) and the subscripts in a formula (which specify the number of each atom within the substance)
- Be able to balance each of the reaction types we have studied:
- Combustion:
- Know the products from the complete burning of hydrocarbons:
- Carbon Dioxide & Water
- Decomposition:
- Be able to write the decomposition of a compound to its component elements:
- Remember the elements that exist as diatomic molecules (H2, N2, O2, F2, Cl2, Br2, I2)
- Synthesis:
- Combining multiple substances into a single compound
- Double Replacement:
- Predict the products of the reaction:
- Be sure to write the products with the correct ratio of positive and negative charges to generate NEUTRAL ionic products
- Be able to use the Solubility Chart to predict the phase of each component involved in a reaction:
- Use phase symbols to specify aqueous solutions (aq), solids (s), liquids (l) and gases (g)
- Single Replacement:
- An elemental substance is combined with a binary compound
STOICHIOMETRY:
- Be able to use the stoichiometric coefficients of a chemical reaction to related one reactant to another and relate reactants to products, or vice versa
- These relationships can come in the form of:
- Mole to mole conversions:
- This is the simplest stoichiometric calculation involving only one step
- Mass to mass conversions:
- Follow the necessary steps: grams to moles, moles to moles, moles back to grams
- Volume to volume conversions: requires density to relate volume to mass
- Know how to calculate Theoretical Yield & Limiting Reagent:
- Understand what the theoretical yield signifies
- Understand the definition of "limiting reagent"
- Be able to calculate theoretical yield based on the limiting reagent
- Identify the reactant that is in EXCESS
- Calculate the amount of reagent in excess that's left over unreacted
