Lecture 18: Gas Laws, PV=nRT
LECTURE 18: Gas Laws, PV=nRT
- Reading: Chp. 11: pp. 359-389
- Suggested Homework: Chapter 11, # 23, 33, 35, 39, 45, 47, 51, 53, 55, 59-63, 67-72, 84-97, 125, 126
- Helpful Resources:
- Propertes of Gases Links to an external site.
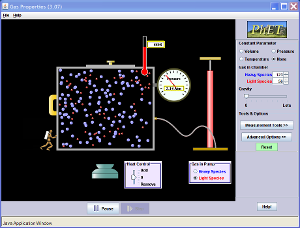
- Simulation of Gas Properties
- Gas Pressure: The Basics Links to an external site.
- Atmospheric Pressure Links to an external site.
- Measuring Atmospheric Pressure and Gas Pressure Links to an external site.
- Boyle's Law Links to an external site.
- Charles' Law Links to an external site.
- Avogadro's Law Links to an external site.
- Gay-Lussac Law Example 1 Links to an external site.
- Gay-Lussac Law Example 2 Links to an external site.
- Rearranging Equations Links to an external site.
- Ideal Gas Law Introduction Links to an external site.
- Ideal Gas Law: PV=nRT Links to an external site. (Khan Academy)
- Ideal Gas Law Example 1 Links to an external site.
- Ideal Gas Law Example 2 Links to an external site.
- Ideal Gas Law Example 3 Links to an external site.
- Ideal Gas Law Example 4 Links to an external site.
- Ideal Gas Law and Density Links to an external site.
- Ideal Gas Law and Molar Mass Links to an external site.
-
The Ideal Gas Law
Links to an external site.
 (Crash Course Chemistry)
(Crash Course Chemistry) -
Ideal Gas Problems
Links to an external site.
 (Crash Course Chemistry)
(Crash Course Chemistry) - Ideal Gas Law: Where did R come from? Links to an external site.
- A wonderfully interesting video on Gases
Links to an external site.